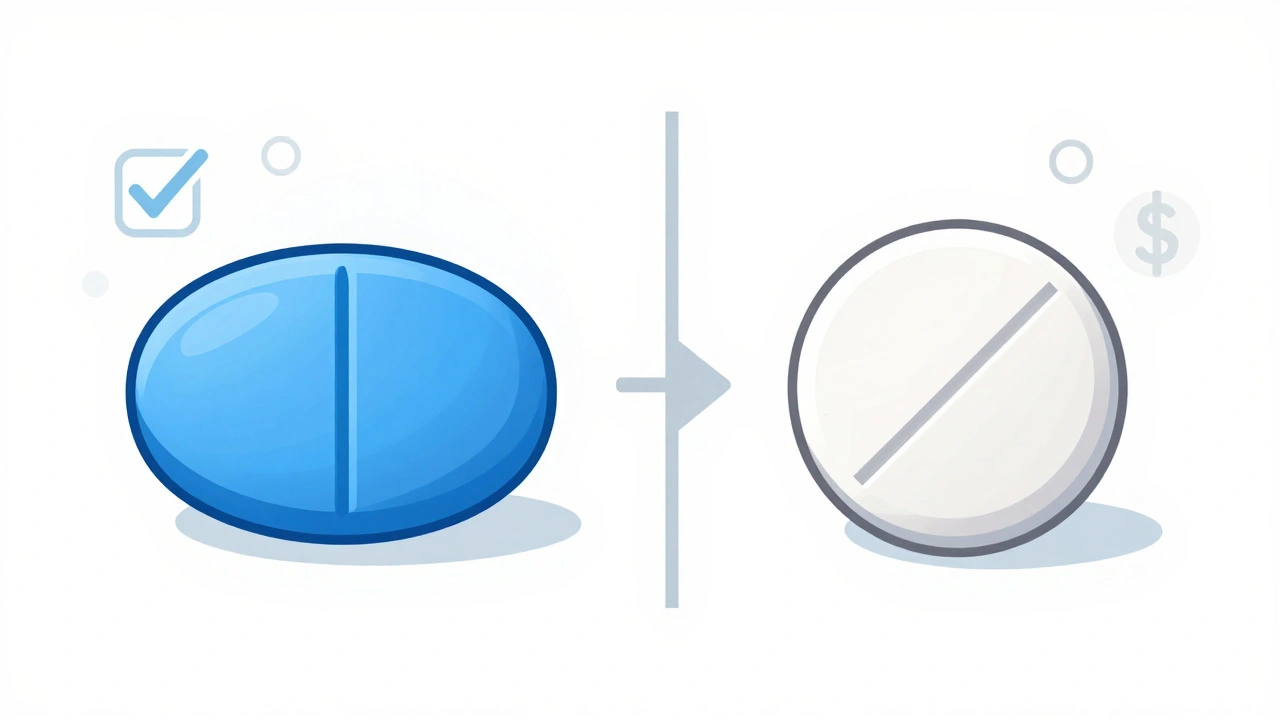
Have you ever picked up your prescription and thought, Wait, this pill looks nothing like the last one? You’re not alone. It’s not a mistake. It’s not a mix-up. And it’s definitely not a fake. That change in color, shape, or size? It’s actually required by law.
Why Generic Pills Don’t Look Like Brand-Name Ones
Generic drugs are just as effective as their brand-name counterparts. They contain the exact same active ingredient, work the same way in your body, and meet the same safety standards. So why do they look different? The answer lies in trademark law - not medicine. In the United States, trademark laws protect the visual identity of products. That includes pills. When a pharmaceutical company creates a new drug, they design its color, shape, and even the imprint on it to make it recognizable. Over time, patients come to associate that look with the medicine they trust. That visual identity becomes part of the brand. But once the patent on that brand-name drug expires, other companies can make the same medicine. That’s where generics come in. The problem? Trademark law says a generic drug can’t look exactly like the original. If it did, it could confuse consumers. Imagine buying a bottle of aspirin and not knowing if it’s Bayer or a generic. That’s a legal no-go. The U.S. Food and Drug Administration (FDA) makes this clear: “Trademark laws in the United States do not allow a generic drug to look exactly like other drugs already on the market.” So generic manufacturers have to change something - anything - to make their version visually distinct. That’s why your generic version of a blue oval pill might now be a white round one with a score line down the middle.What Exactly Changes Between Brand and Generic?
The differences are only in the parts that don’t affect how the drug works. These are called inactive ingredients. They include:- Color: Brand-name drugs often use bright or unique colors to stand out. Generics use different shades - maybe yellow instead of blue, or white instead of pink.
- Shape and size: A brand-name tablet might be oval. The generic could be round or capsule-shaped. Size might be slightly larger or smaller, but not enough to change how it’s absorbed.
- Imprints and scoring: The letters or numbers stamped on pills? Those change too. Even if the brand has a simple “A” on it, the generic might have “G123.” Scoring (the line that lets you break the pill) might be added or removed.
- Flavor and texture: For liquids or chewable tablets, flavorings and binders can differ. That’s why one generic might taste minty while another tastes chalky.
How the System Works: Brand Protection Meets Patient Access
This system wasn’t created to confuse people. It was created to balance two goals:- Let generic companies sell cheaper versions of drugs after patents expire
- Let brand companies keep their unique identity so they can recover R&D costs

Why It Can Be Confusing - and How to Stay Safe
Here’s the real issue: people get confused. You’ve been taking a small, green pill for your blood pressure for years. One day, your refill comes in a large, white pill. Your first thought? Is this the right medicine? You might even wonder if it’s fake. This confusion is real. Studies show that when patients see a change in pill appearance, they’re more likely to skip doses or stop taking their medication altogether. That’s dangerous. Pharmacies know this. That’s why they put clear labels on every bottle. If your pill looks different, the label will say: “Generic version of [Brand Name].” Some even include a note like: “Appearance may vary. Active ingredient unchanged.” The FDA recommends you always check the label against the description on your prescription. Don’t rely on how it looks. Rely on the name on the bottle.What You Can Do When Your Pill Looks Different
Here’s a simple checklist to follow when your generic pill changes:- Check the label. Look for the generic name and the brand name it copies.
- Compare the active ingredient. It should match exactly. For example, “amlodipine” is the active ingredient in both Norvasc and its generics.
- Ask your pharmacist. They’re trained to explain these changes. No question is too small.
- Don’t stop taking it. Unless your doctor says so, keep taking the medicine. The change is legal, safe, and normal.
Is There Any Risk?
No. Not from the medicine itself. The FDA inspects every generic drug manufacturer. They test batches for purity, strength, and consistency. They inspect factories - same as for brand-name drugs. There’s no difference in safety standards. The only risk is psychological: fear of change. And that’s something education can fix. Doctors and pharmacists are trained to explain this. But many patients never get the full story. That’s why confusion persists. The American Medical Association, the FDA, and the World Health Organization all agree: generics are safe, effective, and just as good as brand-name drugs. The only difference? The color.What’s Changing in the Future?
The FDA has noticed the confusion. In recent years, they’ve started recommending that generic manufacturers make their pills as similar as possible to the brand - in size and shape - as long as they still look different enough to avoid trademark infringement. That means you might see fewer wild changes in the future. A brand-name pill that’s oval and blue? The generic might be oval and white. Not a round pill. Not a capsule. Just a different shade. This is a smart compromise. It reduces patient confusion without breaking trademark law. And as more people use generics - and more pharmacies educate patients - the fear around appearance will fade.Bottom Line: Trust the Science, Not the Color
Generic drugs aren’t second-rate. They’re not cheap knockoffs. They’re scientifically identical versions of brand-name drugs - just dressed differently. The reason they look different? Trademark law. Not quality. Not safety. Not effectiveness. You’re not getting less medicine. You’re getting the same medicine, for far less money. And that’s a win. Next time your pill looks different, don’t panic. Check the label. Talk to your pharmacist. Take it. Your body won’t know the difference - and your bank account will thank you.Why do generic drugs look different from brand-name drugs?
Generic drugs look different because U.S. trademark laws require them to have a distinct appearance from brand-name drugs to prevent consumer confusion. While the active ingredient is identical, the color, shape, size, and imprint must be changed so generics don’t copy the visual identity of the original brand.
Are generic drugs less effective because they look different?
No. Generic drugs are required by the FDA to be bioequivalent to brand-name drugs. This means they deliver the same amount of active ingredient into your bloodstream at the same rate. Differences in appearance don’t affect how well the drug works.
Can the different inactive ingredients in generics cause side effects?
Rarely. Inactive ingredients like fillers, dyes, or flavorings are tested for safety. Most people don’t react to them. But if you have a known allergy - like to a specific dye or gluten - talk to your pharmacist. They can check if the generic contains it and suggest an alternative.
Why does my generic pill change shape every time I refill it?
Different generic manufacturers make the same drug. Each one has to create a unique appearance under trademark law. So if your pharmacy switches suppliers, your pill might look different. That’s normal. Always check the label for the generic name and manufacturer.
Should I ask my doctor to prescribe only brand-name drugs?
Only if there’s a specific medical reason. For 90% of medications, generics are just as safe and effective - and cost 80-85% less. Unless your doctor advises otherwise, choosing generics saves money without sacrificing quality.

Ruth Witte
December 9, 2025 AT 09:31Katherine Rodgers
December 10, 2025 AT 08:06Lauren Dare
December 11, 2025 AT 16:10Gilbert Lacasandile
December 11, 2025 AT 21:47Michael Robinson
December 13, 2025 AT 17:31Suzanne Johnston
December 15, 2025 AT 08:30Graham Abbas
December 15, 2025 AT 20:43Haley P Law
December 17, 2025 AT 01:25Steve Sullivan
December 17, 2025 AT 11:40Chris Marel
December 17, 2025 AT 18:13Evelyn Pastrana
December 19, 2025 AT 00:55