
When your doctor hands you a prescription, you might assume the brand name on the bottle is the best option. But here’s the truth: generic medications are just as effective as their brand-name cousins - and often cost 80% less. Yet, despite overwhelming evidence and official endorsements from medical groups, many patients still refuse them. Why? And why do some doctors still prescribe brand-name drugs even when generics are available?
Generics Aren’t Cheap Copies - They’re Exact Matches
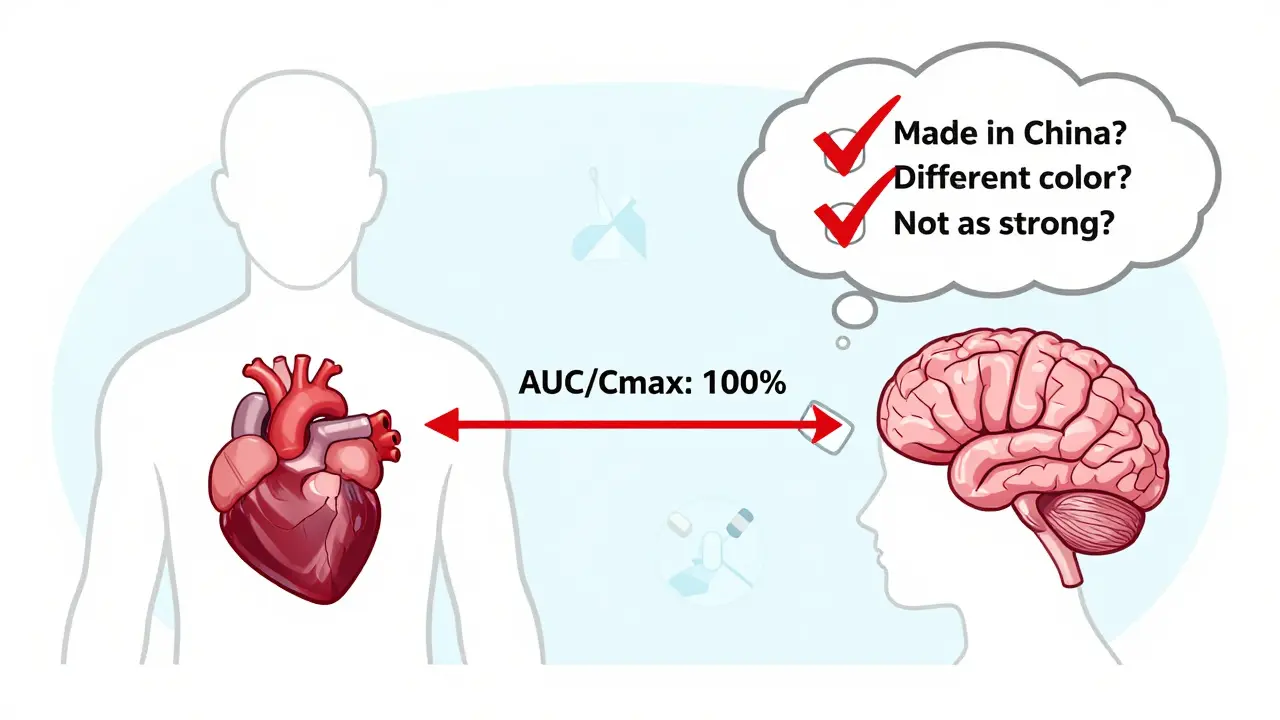
Generic drugs aren’t knockoffs. They contain the exact same active ingredient, in the same strength, and delivered the same way as the brand-name version. The FDA requires them to prove they work identically in your body. That means the amount of medicine entering your bloodstream - measured by AUC and Cmax - must fall within 80% to 125% of the brand-name drug. That’s not close. That’s clinically equivalent.
Manufacturing standards are identical too. The same factories that make brand-name pills often make generics - just under different labels. The FDA inspects over 1,500 generic drug facilities each year, same as brand-name ones. If a plant fails inspection, it’s shut down - no exceptions.
Take lisinopril, for example. The brand-name version, Zestril, costs around $350 a month without insurance. The generic? $4 at Walmart. Same molecule. Same effect. Same side effects. And yet, patients still ask for Zestril. Why?
Doctors Know Generics Work - But They Don’t Always Prescribe Them
The American College of Physicians made it clear in 2016: "Clinicians should prescribe generic medications, if possible." Their recommendation wasn’t based on cost alone. Studies show patients on generics are 6% more likely to stick with their treatment. That’s huge. For someone with high blood pressure or diabetes, better adherence means fewer hospital visits, fewer complications, and longer life.
So why don’t all doctors prescribe generics? A 2016 study of 151 physicians found no clear link between a doctor’s belief in generic cost savings and their prescribing habits. In other words, even doctors who know generics save money don’t always choose them. Why?
One reason: habit. Many doctors were trained to prescribe brand names. It’s what they’ve always done. Another: pressure. About 40% of physicians say patients push them to prescribe generics. Another 41% say patients demand brand names, even when they’re unaffordable. Some patients believe the more expensive option must be better. Others fear the pill looks different - smaller, differently colored, or shaped - and assume it’s weaker.
Even in countries with strong generic use, like Saudi Arabia, only 16% of doctors said they’d use generics in all situations - even though 96% said they understood their therapeutic value. That gap between knowledge and action is real.
The Real Problem Isn’t the Drug - It’s the Perception
Patients aren’t irrational. They’re confused. A 2015 FDA study found people had "mixed perceptions" about generics. They trusted the active ingredient, but worried about the fillers - the inactive ingredients that hold the pill together. Some patients report feeling different after switching, even though the active drug hasn’t changed.
Here’s the catch: those differences aren’t from the medicine itself. They’re from the placebo effect - or the nocebo effect. If you believe a generic won’t work, your body might respond as if it doesn’t. That’s not weakness. That’s psychology.
And it’s not just patients. Pharmacists are often the first to hear complaints. One pharmacist in Sydney told me about a woman who refused her generic metformin because "it didn’t taste right." She’d never tasted the pill before. But she’d heard someone say generics were "made in China" and "unsafe." That story stuck. She stopped taking it. Her blood sugar soared.
The FDA’s "Look Alike Sound Alike" program has helped reduce confusion by 37% since 2018. But education still lags. Most patients don’t know that the same company that makes Lipitor also makes atorvastatin - the generic version. Or that the same factory that makes Nexium makes esomeprazole.

When Generics Aren’t Recommended - And Why
There are exceptions. The FDA lists 15 drugs with a narrow therapeutic index - meaning tiny changes in blood levels can cause serious harm. These include warfarin, levothyroxine, and some anti-seizure drugs. For these, doctors may prefer to stick with one brand to avoid variability.
Even then, studies show most patients do fine switching. A 2020 review of 37 studies on levothyroxine found no significant difference in outcomes between brand and generic. But many doctors still avoid the switch, fearing lawsuits or complaints.
Complex delivery systems are another gray area. Generic inhalers and topical creams can behave differently due to differences in propellants or bases. A 2015 FDA study found asthma patients sometimes felt their generic inhaler didn’t work as well - not because the medicine was weaker, but because the spray felt different. That’s a usability issue, not a safety one.
Why Your Doctor Might Not Push Generics - And What You Can Do
Doctors aren’t paid to push generics. They’re paid to treat you. If you’re struggling to afford your meds, they might not know. Or they might assume you’re fine because you didn’t complain.
Here’s what you can do:
- Ask: "Is there a generic version?" - Don’t wait for them to bring it up.
- Ask: "Will this work the same as the brand?" - Most will say yes.
- Ask: "Can we try the generic?" - Frame it as a trial, not a demand.
- Check your pharmacy’s price. Many generics cost less than your copay. Some are even free.
One internist in Melbourne told me about a patient who refused generic lisinopril for two years. He finally switched after his rent went up. Within a month, his blood pressure improved. He told the doctor: "I thought the $350 pill was magic. Turns out, the $4 one just worked better because I actually took it."
The Bigger Picture: Generics Save Lives - And Billions
In the U.S., generics make up 90% of prescriptions but only 23% of drug spending. That’s $250 billion saved annually. In Medicare Part D alone, if new prescriptions matched overall dispensing rates, beneficiaries could save $17.3 billion a year.
And it’s not just the U.S. In Canada, after generic versions of blood pressure drugs hit the market, prescriptions jumped - but so did ER visits. At first, that looked bad. But researchers later realized: patients were skipping doses because they couldn’t afford the brand. Once generics arrived, they could afford to take them - and their health improved.
Global generic drug sales are projected to hit nearly $600 billion by 2028. Biosimilars - generic versions of complex biologic drugs - are starting to enter the market. This isn’t a trend. It’s the future.
Final Thought: It’s Not About the Pill - It’s About Trust
The science is settled. Generics work. They’re safe. They’re cheaper. The only thing standing in the way is perception.
Doctors know this. Patients know this - deep down. But trust doesn’t come from data. It comes from conversation. From being heard. From knowing your doctor isn’t trying to save money on your back - they’re trying to help you stay healthy without going broke.
If you’ve ever refused a generic because you thought it was "lesser," ask yourself: What’s the real reason? Is it the pill? Or the story you’ve been told?
Are generic medications as safe as brand-name drugs?
Yes. The FDA requires generics to meet the same strict standards as brand-name drugs for quality, strength, purity, and potency. They must prove bioequivalence - meaning they deliver the same amount of active ingredient into your bloodstream at the same rate. The same manufacturing rules apply to both. Generic drugs are not "second-rate." They’re the same medicine, just without the marketing costs.
Why do some doctors still prescribe brand-name drugs?
Many doctors prescribe brand names out of habit, patient pressure, or lack of awareness about cost differences. Some worry about patient complaints if they switch, even though studies show no difference in outcomes. Others are influenced by pharmaceutical reps or outdated training. But research shows that when doctors get proper education on generics, their prescribing rates increase by up to 23% within six months.
Can I trust generics made overseas?
Yes. Over half of all generic drugs sold in the U.S. are made overseas, mostly in India and China. But every facility - whether in Ohio or Mumbai - must pass the same FDA inspections. The FDA conducts over 1,000 inspections of domestic and 500 of foreign facilities each year. If a plant fails, it’s blocked from selling to the U.S. market. Location doesn’t determine safety. Regulation does.
Why do generic pills look different from brand-name ones?
By law, generics can’t look identical to brand-name drugs - that would violate trademark rules. So they’re different colors, shapes, or sizes. But that doesn’t affect how they work. The active ingredient is the same. The FDA has a "Look Alike Sound Alike" program to reduce confusion, and it’s cut patient errors by 37% since 2018. If you’re unsure, ask your pharmacist to explain the change.
Do generics take longer to work?
No. Generics must prove they work at the same speed as the brand-name version. This is measured through bioequivalence studies that track how quickly the drug enters your bloodstream (Cmax) and how long it stays there (AUC). If a generic doesn’t match within strict FDA limits, it’s rejected. There’s no delay in effectiveness.
What if I feel different after switching to a generic?
You’re not imagining it - but it’s likely not the drug. Some people notice differences in how the pill feels or tastes, or they worry it won’t work. This can trigger a psychological response called the nocebo effect. If you feel worse, talk to your doctor. It could be a reaction to inactive ingredients, but it’s rare. Most often, it’s anxiety. If needed, your doctor can switch you back - but give it a few weeks first. Your body might just need time to adjust.
Are there any drugs where generics aren’t recommended?
Yes - but only 15 drugs on the FDA’s list have a narrow therapeutic index, meaning tiny changes in blood levels can cause serious problems. These include warfarin, levothyroxine, phenytoin, and some epilepsy drugs. Even then, most patients switch safely. Doctors may choose to stick with one brand for consistency, but it’s not because generics are unsafe - it’s about minimizing variables in sensitive cases.
Will my insurance cover generics?
Almost always - and usually at a much lower cost. Many insurance plans have lower copays for generics. Some even require you to try the generic first before covering the brand. In some cases, the generic costs less than your copay. Always check with your pharmacy. You might be paying more than you need to.
How can I tell if a drug is generic?
Look at the label. Generic drugs are listed by their active ingredient name - like "atorvastatin" instead of "Lipitor." The manufacturer name will be different too - often a company you’ve never heard of. Your pharmacist can confirm if what you’re getting is generic. You can also search the drug name on the FDA’s website or ask your doctor to write "dispense as written" if you prefer the brand.
Can I switch back to the brand if I don’t like the generic?
Yes. If you feel the generic isn’t working or causes side effects, talk to your doctor. They can write a new prescription for the brand-name version. But don’t assume it’s the drug - give it time. Most people adjust within a few weeks. And remember: the only thing that changes is the price and the look. The medicine inside is the same.

Tim Goodfellow
December 20, 2025 AT 01:10Generics are the unsung heroes of modern medicine. That $4 lisinopril? Same molecule as Zestril. Same blood pressure control. Same chance of saving your life. The only difference? Your wallet doesn’t cry when you pay for it. Why are we still acting like generics are some kind of pharmacy loophole? They’re not. They’re the science we already proved works.
mark shortus
December 21, 2025 AT 21:39My grandma switched to generic metformin last year. Said the pill looked ‘too small’ and ‘suspicious.’ Two weeks later? She was like ‘Wait… I didn’t feel like a zombie after lunch anymore?’ Turns out the brand made her nauseous. The generic? Clean. Smooth. She now calls it ‘the little white knight.’
Elaine Douglass
December 22, 2025 AT 20:18I used to think generics were sketchy until my insurance forced me to switch. I was scared. But after a month? My cholesterol dropped. My doctor said I was doing great. I just wish someone had told me this sooner. No drama. No side effects. Just results.
Takeysha Turnquest
December 23, 2025 AT 05:41They told us the pill was the same. But what if the soul of the medicine is in the brand? The packaging. The history. The trust built over decades. When you take a generic, are you taking the drug-or just the chemical ghost of something once revered? The body remembers more than bioequivalence charts.
Alex Curran
December 23, 2025 AT 23:22My pharmacist in Melbourne once showed me a chart: 90% of prescriptions are generic, 23% of total spending. That’s $250 billion saved every year in the US alone. We’re not saving pennies here. We’re saving lives by making treatment accessible. And yet people still panic because the pill is blue instead of green.
Lynsey Tyson
December 24, 2025 AT 07:53I get why people are nervous. I was too. But my doctor sat with me for 20 minutes and explained how the FDA checks every batch. Now I ask for generics every time. It’s not about being cheap. It’s about being smart. And honestly? I feel better knowing I’m not wasting money on marketing.
Edington Renwick
December 26, 2025 AT 02:41Let’s be real. Most doctors prescribe brand names because they’re lazy. They don’t want to explain why the generic looks different. They don’t want to deal with patients who think it’s ‘fake.’ So they just write the expensive one. Convenience over care. That’s the real problem.
Sarah McQuillan
December 26, 2025 AT 23:09Generics are made in China. You know what else is made in China? Phones. Toys. Tires. And yet somehow the pills are the only thing we’re terrified of? If your phone works, why not your blood pressure medicine? The FDA doesn’t care where it’s made. They care if it works. And it does.
Aboobakar Muhammedali
December 28, 2025 AT 22:39I saw my cousin in Mumbai take generic insulin. Same as the US brand. Same price. Same results. He’s been on it for 5 years. No complications. No issues. The fear is not about the drug. It’s about the story we tell ourselves. We’ve been sold a myth that expensive = better. But biology doesn’t care about price tags.
Laura Hamill
December 30, 2025 AT 11:50THEY’RE MAKING GENERICS IN CHINA WITH LABOR CAMP DUST AND RATS IN THE PACKAGING. I SAW A VIDEO. THE PILLS ARE MADE BY PRISONERS. THE FDA IS A LIE. MY FRIEND’S COUSIN’S NEPHEW GOT SICK FROM A GENERIC. I KNOW THIS BECAUSE I READ IT ON A FACEBOOK GROUP. DON’T BE A SHEEP. STICK TO BRANDS. THEY’RE PROTECTED. THE GOVERNMENT CAN’T TOUCH THEM. 💀
Alana Koerts
December 31, 2025 AT 22:19It’s not that generics don’t work. It’s that the entire system is rigged. Why do we still have brand-name drugs if generics are identical? Because pharma needs to profit. The FDA’s ‘80-125% bioequivalence’ range is a joke. That’s a 45% variance. That’s not equivalent. That’s gambling. And we’re the pawns.
Dikshita Mehta
January 2, 2026 AT 11:31I work in a clinic in Delhi. We use generics for everything. Blood pressure, diabetes, antibiotics. We’ve tracked patients for years. No difference in outcomes. Some even do better because they can afford to take them daily. The real issue isn’t science. It’s access. And education. We need more doctors explaining this, not just prescribing.
Gloria Parraz
January 3, 2026 AT 17:36My patient who refused generic lisinopril for two years? He finally switched when his rent doubled. Said he thought the $350 pill was magic. Turned out the $4 one worked better because he actually took it. That’s the real story. Not chemistry. Consistency. Humanity.
Guillaume VanderEst
January 5, 2026 AT 12:46I used to think generics were a compromise. Then I got hit with a $2,000 bill for a brand-name statin. Switched to atorvastatin. Same pill. Same results. Saved $1,800. Now I tell everyone. It’s not about trust in the pill. It’s about trust in yourself to make the smart choice.
Kelly Mulder
January 5, 2026 AT 21:43While I appreciate the sentiment, the premise is fundamentally flawed. The FDA’s bioequivalence standards are not only arbitrary but statistically porous-allowing for a 20% variance in absorption rates. This is not equivalence. It is tolerance. And when one considers the pharmacokinetic variability across ethnic populations, the notion that a generic manufactured in Mumbai is ‘identical’ to one produced in New Jersey is not merely optimistic-it is dangerously naive. The placebo effect may be psychological, but the consequences of suboptimal absorption are physiological-and irreversible. This article reads like a pharmaceutical industry white paper disguised as public health advocacy.