Switching from brand-name phenytoin to a generic version might seem like a simple cost-saving move - but for patients taking this drug, it can be risky. Phenytoin isn’t like most medications. Even small changes in blood levels can lead to seizures or serious toxicity. That’s why therapeutic drug monitoring isn’t optional when switching between phenytoin formulations - it’s essential.
Why Phenytoin Is Different
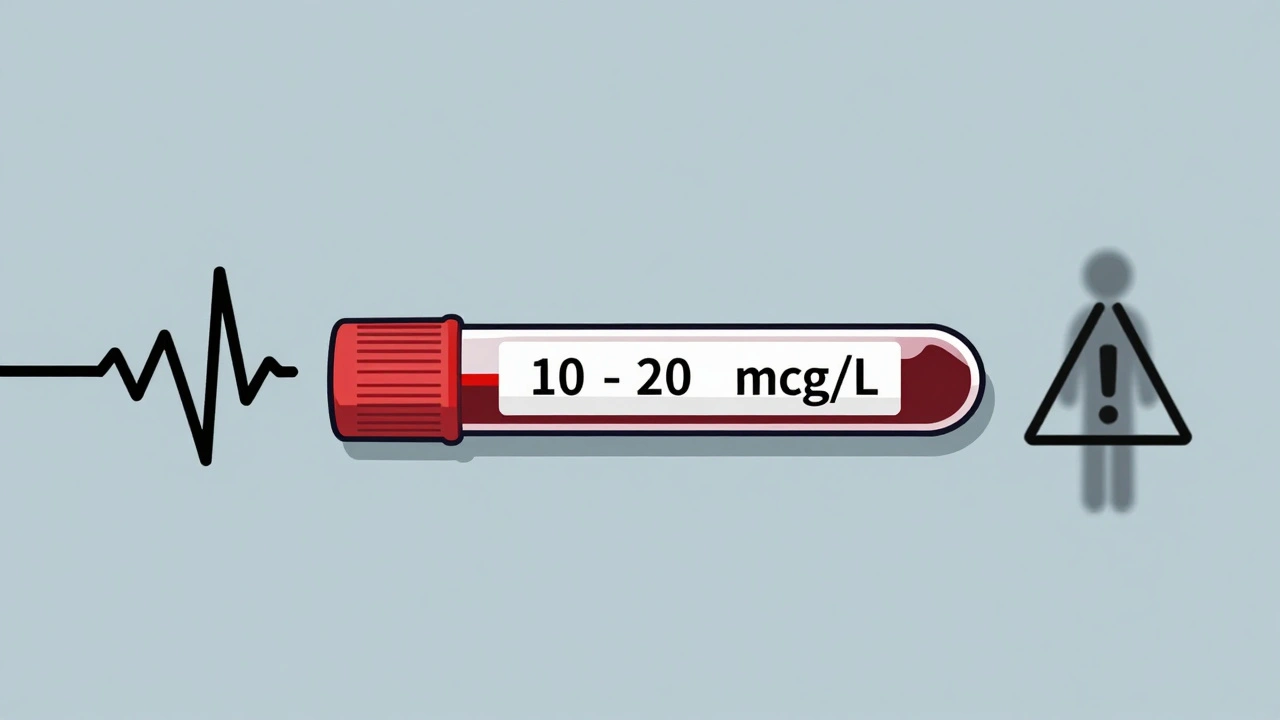
Phenytoin has been used since the 1930s to control seizures, and it still works. But it’s tricky. It has a very narrow therapeutic window: 10 to 20 mcg/mL. Go below 10, and seizures might return. Go above 20, and you risk confusion, loss of coordination, or even coma. At levels over 50 mcg/mL, death becomes possible. What makes it worse is how the body handles the drug. At low doses, phenytoin is cleared predictably. But as levels rise, the enzymes that break it down get overwhelmed. That’s called zero-order kinetics. A tiny increase in dose - say, 25 to 50 mg - can cause a huge spike in blood levels. There’s no safety buffer. Add to that: phenytoin is 90-95% bound to proteins in the blood. Only the small unbound portion actually works. So if a patient has low albumin (common in elderly people, those with liver disease, or malnutrition), their total phenytoin level might look normal - but the active, unbound part could be dangerously high.Generic Substitutions: The Hidden Risk
Generic drugs must meet FDA standards for bioequivalence. That means the amount of drug absorbed should be within 80-125% of the brand-name version. Sounds fine, right? Not for phenytoin. That 45% range - from 80% to 125% - is huge when your therapeutic window is only 10 mcg/mL wide. A patient stable at 15 mcg/mL on one generic could drop to 12 mcg/mL or jump to 18 mcg/mL after switching to another. Both changes can be dangerous. One might trigger a seizure. The other could cause toxicity. Studies and guidelines from NHS Tayside, the American Academy of Family Physicians, and others agree: when switching phenytoin formulations - whether from brand to generic, or between two generics - you must monitor blood levels.When to Check Levels
Timing matters. Phenytoin takes time to reach steady state. Don’t test too early.- Check a level 2-3 days after starting or changing dose - just to make sure metabolism isn’t abnormal.
- Check again at 5-7 days - this is when steady state is usually reached.
- After switching formulations, check a trough level (just before the next dose) at 5-10 days.
- If given intravenously, wait 2-4 hours after a loading dose before testing.
- For oral doses, wait 12-24 hours.
Special Cases: Who Needs Extra Care
Not everyone needs frequent monitoring. But some patients are at higher risk:- Older adults - often have lower albumin, slower metabolism.
- People with liver disease - phenytoin is metabolized in the liver.
- Those with kidney failure - affects protein binding and clearance.
- Pregnant women - metabolism changes during pregnancy.
- Patients on multiple medications - many drugs interfere with phenytoin.
Correcting Levels for Low Albumin
If a patient has low albumin, the total phenytoin level can be misleading. A “normal” level of 14 mcg/mL might actually mean a toxic unbound level. The formula used to estimate the corrected level is:Corrected phenytoin = Measured level / [(0.9 × Albumin g/L) / 42 + 0.1]
But here’s the catch: this formula is just a rough estimate. It’s based on population averages. In real patients, especially those who are critically ill or malnourished, it can be off. The best approach? If albumin is below 30 g/L, check the free phenytoin level instead. That tells you exactly how much active drug is in the blood. Many hospitals now offer this test - and it’s worth requesting if the patient seems off despite a “normal” total level.Long-Term Monitoring Beyond Blood Levels
Phenytoin doesn’t just affect your brain. Long-term use causes other problems:- Gingival hyperplasia (swollen gums)
- Hirsutism (excess hair growth)
- Thickened facial features
- Vitamin D deficiency
- Folic acid deficiency
- Osteomalacia (soft bones)
- Peripheral neuropathy
- Baseline: CBC, liver function, electrolytes, vitamin D, calcium, phosphate
- Every 2-5 years: repeat bone health tests and CBC
- For Asian patients (Han Chinese or Thai): test for HLA-B*1502 gene before starting - this variant increases risk of severe skin reactions
What to Do When Switching Formulations
Here’s a simple, practical plan:- Before switching, get a trough level.
- Record how the patient is feeling - seizure frequency, dizziness, tremors, gum swelling.
- Switch to the new formulation.
- Check another trough level at 5-10 days.
- If the level is outside 10-20 mcg/mL, adjust dose and recheck in 5-7 days.
- If the patient feels worse - even if levels look fine - check free phenytoin and review all other medications.
Bottom Line
Generic phenytoin is cheaper. But cost savings shouldn’t come at the cost of safety. For this drug, therapeutic drug monitoring isn’t a nice-to-have. It’s a must. Patients on phenytoin need consistent formulations. If a switch happens, monitor closely. Test levels. Watch for side effects. Check albumin. Consider free phenytoin. Track bone and vitamin health. This isn’t about being overly cautious. It’s about understanding how phenytoin works - and why even small changes can have big consequences. For people who depend on this drug to prevent seizures, there’s no room for guesswork.Is it safe to switch between generic phenytoin brands?
Switching between different generic brands of phenytoin carries risk. Even though they meet FDA bioequivalence standards, phenytoin’s narrow therapeutic window and non-linear metabolism mean small differences in absorption can cause seizures or toxicity. Always check serum levels before and after switching, and monitor the patient closely for clinical changes.
Do I need to check phenytoin levels if I’ve been on it for years?
If your dose and formulation haven’t changed, and you’re stable with no side effects, routine monitoring isn’t always needed. But if you start a new medication, develop liver or kidney problems, become pregnant, or have low albumin, check your level. Also, monitor bone health and vitamin D every few years.
What if my total phenytoin level is normal but I still have side effects?
If you have symptoms like dizziness, confusion, or tremors despite a normal total level, ask for a free phenytoin test. Low albumin - common in older adults or those with chronic illness - can cause high levels of active drug even when total levels look fine. Your clinical symptoms matter more than the number on the report.
Can I take phenytoin with other medications?
Many drugs interact with phenytoin. Antibiotics like metronidazole, antifungals like fluconazole, and heart drugs like amiodarone can raise phenytoin levels and cause toxicity. Alcohol, rifampin, and seizure drugs like carbamazepine can lower it and trigger seizures. Always review all medications - including over-the-counter and herbal products - with your doctor or pharmacist before starting or stopping anything.
How often should I get my bone health checked on phenytoin?
Patients on long-term phenytoin should have bone health checked every 2-5 years. This includes vitamin D, calcium, phosphate, and alkaline phosphatase levels. Phenytoin can reduce vitamin D and lead to weak bones. If you’re over 50 or have a history of fractures, talk to your doctor about a bone density scan.
Why is phenytoin monitoring not needed for all antiepileptic drugs?
Most newer antiepileptic drugs have wider therapeutic windows and linear pharmacokinetics. Their levels don’t spike dangerously with small dose changes, and they’re less affected by protein binding or liver function. Phenytoin is one of the few older drugs with non-linear metabolism - making it uniquely sensitive to small changes. That’s why it needs special attention.

Louise Girvan
December 1, 2025 AT 14:41Jaswinder Singh
December 2, 2025 AT 02:41Linda Migdal
December 3, 2025 AT 16:59Tommy Walton
December 4, 2025 AT 04:41Sean McCarthy
December 4, 2025 AT 09:48Courtney Co
December 5, 2025 AT 19:31Priyam Tomar
December 6, 2025 AT 11:23Irving Steinberg
December 6, 2025 AT 21:45Lydia Zhang
December 8, 2025 AT 16:48soorya Raju
December 9, 2025 AT 16:26Lucinda Bresnehan
December 11, 2025 AT 14:03Kshitij Shah
December 13, 2025 AT 01:40Bee Floyd
December 13, 2025 AT 19:16Jeremy Butler
December 14, 2025 AT 14:33