For decades, proving that a generic drug works the same as the brand-name version meant putting healthy volunteers through blood draws, frequent visits, and weeks of clinical monitoring. These in-vivo bioequivalence studies cost up to $2 million each and took months to complete. But now, a smarter, faster approach is taking over: in-vitro methods that predict how a drug behaves in the body-without ever giving it to a person.
What Is IVIVC and Why Does It Matter?
IVIVC stands for In Vitro-In Vivo Correlation. It’s a scientific model that links what happens to a drug in a test tube (in vitro) to what happens inside the human body (in vivo). Specifically, it connects how quickly a pill dissolves in a lab setting to how fast and how much of the drug actually gets into the bloodstream. This isn’t just academic. If you can prove that dissolution in a beaker reliably predicts blood levels in a patient, you can skip the human trials. That’s called a biowaiver. Regulatory agencies like the FDA and EMA now accept these waivers for certain generic drugs, saving companies millions and speeding up access to affordable medicines. The FDA first laid out the rules in 1996, but it wasn’t until the 2014 revision that the framework became truly actionable. Today, IVIVC is the gold standard for complex drug products-especially extended-release tablets and capsules-where simple chemical equivalence isn’t enough to guarantee safety and effectiveness.The Four Levels of IVIVC: Not All Correlations Are Equal
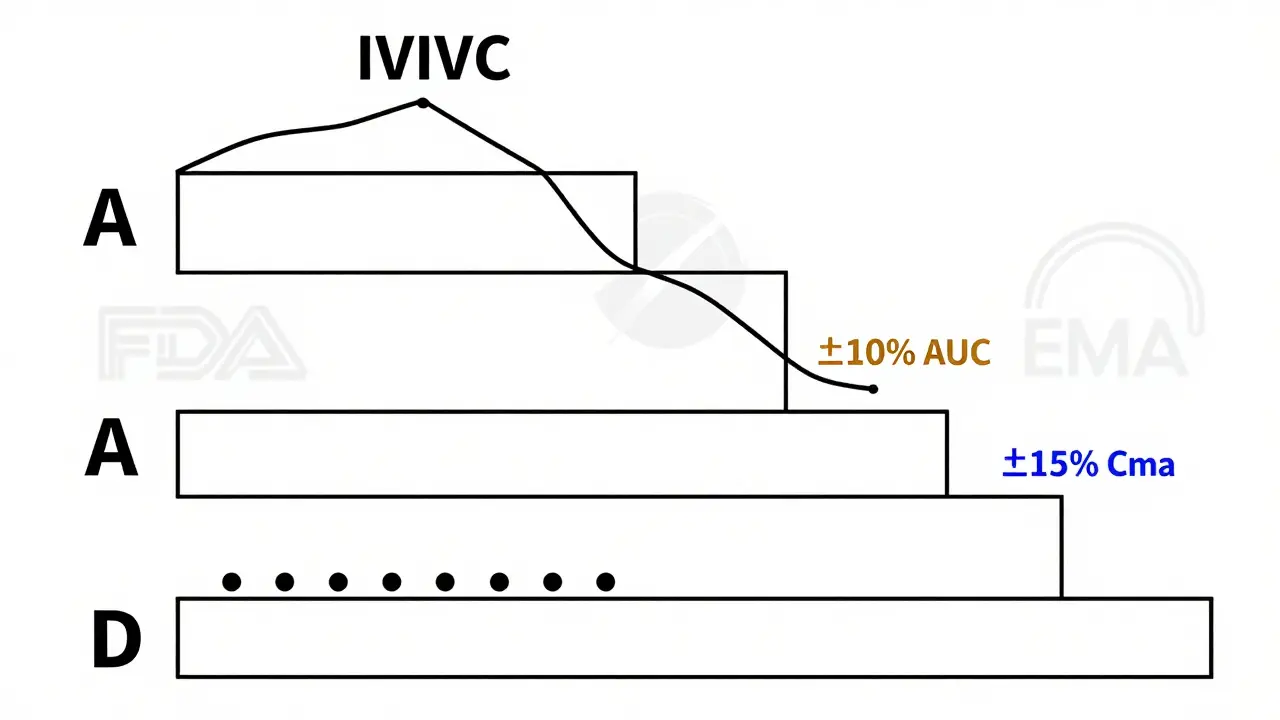
Not every IVIVC model is created equal. The FDA classifies them into four levels, and only some qualify for biowaivers.- Level A: The dream scenario. It’s a point-by-point match between dissolution at every time point and drug absorption in the body. Think of it like a perfect mirror: if the pill dissolves 30% in 1 hour in the lab, the blood level rises exactly as predicted. This requires a correlation coefficient (R²) above 0.95 and a slope near 1.0. Only Level A models are widely accepted for full biowaivers.
- Level B: Uses averages instead of exact matches. It links the average time it takes for the drug to dissolve to the average time it stays in the body. Useful, but not precise enough to replace human studies for most cases.
- Level C: A single-point link-for example, how much dissolves in 1 hour versus peak blood concentration (Cmax). Limited in scope. Multiple Level C models (linking several dissolution points to multiple PK parameters) can sometimes work, but regulators are cautious.
- Level D: No correlation at all. Just a vague association. Not accepted for waivers.
Why Dissolution Testing Isn’t Just Stirring a Pill in Water
You might think dissolution testing is as simple as dropping a pill in a beaker of water and watching it dissolve. It’s not. Real-world absorption happens in the stomach and intestines-where pH changes, bile salts are present, and food affects how drugs move. That’s why biorelevant dissolution testing is now essential. Instead of plain water, labs use fluids that mimic the human gut: simulated gastric fluid (pH 1.2), intestinal fluid (pH 6.8), and even solutions with bile salts and phospholipids. These conditions make the test far more predictive. A 2019 study from the University of Maryland showed that traditional dissolution methods failed to distinguish between two similar extended-release formulations-until biorelevant media revealed one released drug too slowly to be effective. That’s the kind of difference that can mean the difference between a safe generic and a dangerous one.When IVIVC Saves Millions-And When It Costs More
The math is clear: one bioequivalence study with 24 volunteers costs between $500,000 and $2 million. An IVIVC program might cost $800,000 to $1.5 million upfront-but it can replace 5 to 10 studies over the drug’s lifecycle. Teva saved over $10 million on their extended-release oxycodone generic by developing a Level A IVIVC. Instead of running five post-approval bioequivalence studies for minor formulation tweaks, they just ran a dissolution test. The FDA approved each change within weeks. But here’s the catch: IVIVC isn’t cheap to get right. It requires:- 3-5 different formulations with varying release rates
- 3-4 separate pharmacokinetic studies with 12-24 volunteers each
- At least 12 blood draws per subject over 24-72 hours
- Specialized software and statisticians who understand both dissolution and pharmacokinetics
Who’s Using IVIVC-and Who Isn’t
IVIVC adoption is growing, but it’s not evenly distributed. Only about 15% of pharmaceutical companies have the in-house expertise to develop these models. The big players-Teva, Sandoz, Mylan, Sun Pharma, and Lupin-have dedicated teams. Smaller companies often outsource to specialized CROs like Alturas Analytics or Pion, which report success rates of 60-70% when brought in early. The FDA approved just 15% of IVIVC submissions in 2018. By 2022, that number jumped to 42%. Why? Better science. More experience. And stricter guidance. Still, failure rates remain high. In 2023, the FDA reviewed 127 IVIVC submissions. Of those, 64% were rejected because the dissolution conditions didn’t reflect real human physiology. Another 28% failed because the model wasn’t properly validated across multiple scenarios.IVIVC vs. BCS: Two Paths to a Biowaiver
For immediate-release tablets, there’s a simpler route: the Biopharmaceutics Classification System (BCS). If a drug is:- Highly soluble (Class I or III)
- Highly permeable (Class I or II)
- Doesn’t have a narrow therapeutic index
What’s Next? AI, Machine Learning, and New Frontiers
The future of IVIVC isn’t just better models-it’s smarter ones. In 2024, the FDA and EMA held a joint workshop on machine learning-enhanced IVIVC. Researchers are now training algorithms to predict absorption patterns from dissolution data, using thousands of historical datasets. The goal? Reduce the number of formulations and volunteer studies needed. One pilot project reduced required in vivo data by 40% using AI to identify the most informative dissolution profiles. Regulators are also expanding IVIVC beyond oral drugs. Draft guidance for topical products was released in June 2023. The EMA is exploring IVIVC for injectables and ophthalmic formulations. Implantable devices and vaginal inserts are next on the list. But even with AI, the core rule hasn’t changed: if the test doesn’t reflect real human biology, it’s not valid.
Real-World Risks: When IVIVC Gets It Wrong
There’s a reason regulators are cautious. In 2021, a generic extended-release antidepressant received approval based on a multiple Level C IVIVC. Within months, reports surfaced of patients experiencing withdrawal symptoms. The generic dissolved too slowly under fasting conditions, leading to subtherapeutic blood levels. The issue? The IVIVC model didn’t account for food effects. The company had only tested the drug in fasted volunteers. Real patients took it with meals. Dr. Jennifer Dressman of Goethe University Frankfurt warned in 2023: “Multiple Level C correlations, while easier to develop, often fail to capture the full complexity of drug release and absorption.” That’s why the EMA now requires IVIVC models to be validated across multiple physiological conditions-fasted, fed, acidic, and with bile salts.How to Know If IVIVC Is Right for Your Product
Ask yourself these questions:- Is this an extended-release, modified-release, or complex dosage form? → IVIVC is likely needed.
- Is the drug highly soluble and rapidly absorbed? → Try BCS first.
- Is the therapeutic index narrow (like warfarin or digoxin)? → Stick with in vivo studies.
- Do you have access to pharmacokinetic experts and biorelevant dissolution equipment? → If not, partner with a CRO.
- Are you planning multiple post-approval changes? → IVIVC pays off over time.
Final Thoughts: The Future Is Predictive, Not Experimental
The era of testing drugs on hundreds of healthy volunteers is fading. The future belongs to predictive science. IVIVC is no longer a niche technique-it’s becoming the standard for complex generics. By 2027, McKinsey & Company predicts that 35-40% of all modified-release generic approvals will rely on IVIVC, up from just 22% in 2022. Biorelevant dissolution will be standard for 75% of new submissions. The message is clear: if you’re in generic drug development, you need to understand IVIVC. Not because it’s trendy-but because it’s the only way to bring safe, effective, affordable medicines to market without unnecessary human testing.What is the main purpose of IVIVC in generic drug development?
The main purpose of IVIVC is to establish a scientific link between how a drug dissolves in a lab setting and how it behaves in the human body. This allows regulators to approve generic versions without requiring expensive and time-consuming human bioequivalence studies, as long as the in vitro dissolution profile reliably predicts in vivo performance.
Can IVIVC be used for all types of drugs?
No. IVIVC works best for oral extended-release and modified-release products where absorption is complex. It’s not suitable for immediate-release drugs that dissolve quickly (those use BCS biowaivers), drugs with narrow therapeutic indexes (like warfarin), or products with nonlinear pharmacokinetics. Injectable, ophthalmic, and topical products are being studied, but regulatory acceptance is still limited.
Why do so many IVIVC submissions get rejected by the FDA?
Most rejections happen because the dissolution test doesn’t reflect real human conditions. Common issues include using plain water instead of biorelevant fluids, testing too few formulations, or not validating the model under fed/fasted conditions. The FDA requires models to predict AUC within ±10% and Cmax within ±15%-many fail to meet this bar.
Is IVIVC cheaper than running bioequivalence studies?
Upfront, IVIVC can cost $1-1.5 million. But it replaces 5-10 human bioequivalence studies, each costing $500,000-$2 million. Over the drug’s lifecycle, IVIVC saves millions and reduces approval timelines by 6-12 months. The cost only makes sense for products with multiple post-approval changes or long-term commercial potential.
What’s the difference between BCS and IVIVC biowaivers?
BCS biowaivers apply to immediate-release drugs that are highly soluble and highly permeable. You only need to prove dissolution is fast and complete. IVIVC is for complex products-like extended-release tablets-where dissolution rate controls absorption. IVIVC requires detailed modeling and multiple formulations; BCS doesn’t.
Can machine learning improve IVIVC accuracy?
Yes. Machine learning models are being used to analyze thousands of historical dissolution and pharmacokinetic datasets to identify patterns humans might miss. Early results show AI can reduce the number of required in vivo studies by 30-40% and improve model predictability. Both the FDA and EMA are open to these approaches if they’re transparent and scientifically sound.

Madhav Malhotra
January 10, 2026 AT 12:54This is so cool! In India, we’re seeing more generic meds reach rural clinics thanks to these methods. Less cost = more people helped. 🙌
Christian Basel
January 11, 2026 AT 07:15IVIVC is essentially a glorified dissolution curve fitting exercise with a fancy acronym. The FDA’s acceptance is less about science and more about bureaucratic expediency. Level A? More like Level A for lazy regulators.
Roshan Joy
January 12, 2026 AT 12:19Really appreciate how you broke down the levels. Level A being the gold standard makes sense - it’s like having a perfect GPS for drug absorption. But I wonder how many labs outside the US/EU even have the equipment for biorelevant media? 🤔
Sean Feng
January 12, 2026 AT 19:36So you're telling me we're replacing human tests with machines that don't even replicate real gut conditions? That's just putting lipstick on a pig
Adewumi Gbotemi
January 12, 2026 AT 23:46This is good stuff. In Nigeria, we need cheaper drugs. If this helps make that happen, then it’s a win. Just hope they test it right.
Priya Patel
January 13, 2026 AT 00:28OMG I just realized this is why my generic Xanax didn’t work last month 😱 I thought it was me… turns out maybe the IVIVC model skipped the fed condition. YIKES.
Alfred Schmidt
January 13, 2026 AT 10:21Stop pretending this is science! You’re not predicting anything-you’re just guessing based on a few lab conditions that have zero relevance to how real people take their meds. And you call this progress? Pathetic.
Priscilla Kraft
January 13, 2026 AT 17:54Love this breakdown! 🌟 I work in a CRO and we’ve seen firsthand how biorelevant media catches what water can’t. One time, we caught a formulation that looked perfect in plain buffer but dissolved 40% slower in fasted intestinal fluid. Saved a patient from underdosing. That’s the real win.
Vincent Clarizio
January 14, 2026 AT 00:11Let’s not kid ourselves-this whole IVIVC paradigm is a philosophical shift from empirical observation to algorithmic reductionism. We’re moving away from the embodied experience of pharmacokinetics-the messy, variable, human reality of digestion, metabolism, and individual biochemistry-and replacing it with a statistical abstraction that assumes homogeneity where none exists. The FDA doesn’t want to test people; they want to avoid liability. And industry? They want to maximize ROI while minimizing human exposure. So we engineer a model that fits the data we have, not the reality we don’t. This isn’t innovation-it’s institutionalized epistemic laziness dressed up as efficiency. And the worst part? We’re celebrating it as progress.
Sam Davies
January 14, 2026 AT 16:51Oh wow, another 15-page whitepaper masquerading as a blog post. Next you’ll tell me AI will predict my coffee’s effect on my cortisol levels. Level A? More like Level A for people who think R² > 0.95 is a magic spell.
Alex Smith
January 16, 2026 AT 10:54Actually, this is a great example of how science can be both efficient AND ethical. You don’t need to stick 24 people in a clinic for a month to test a pill. But you DO need to get the damn dissolution right. If you’re skipping fed/fasted conditions, you’re not being smart-you’re being reckless. And yeah, AI helps, but only if you feed it real data. No magic.
Michael Patterson
January 17, 2026 AT 00:23uuhh so like… IVIVC is good but like… what if the dissolution test is wrong? like i read somewhere that like 60% of the time the models fail because they used the wrong buffer or something? like why dont they just test on people? its easier??