When you pick up a generic prescription, you might wonder: Is this really the same as the brand-name drug? It’s a fair question. After all, the price is lower, the packaging looks different, and you’ve probably heard rumors that generics aren’t as strong. But here’s the truth most people miss: the 80-125% rule isn’t about how much active ingredient is in the pill. It’s about how your body absorbs it.
What the 80-125% Rule Actually Measures
The 80-125% range isn’t a tolerance for how much drug is in the tablet. It’s a statistical boundary for how fast and how completely your body takes in the medicine. This is called bioequivalence. For a generic drug to be approved, its absorption profile - measured in your bloodstream - must match the brand-name version within this range.
Two key numbers are tracked: AUC (area under the curve), which tells you how much of the drug gets into your system over time, and Cmax, which shows how quickly it peaks. If the generic’s AUC and Cmax fall within 80-125% of the brand’s, it’s considered bioequivalent. That doesn’t mean the generic could have 25% less or 25% more drug. It means your body processes it so similarly that the difference won’t affect how well it works.
The FDA uses a 90% confidence interval to make sure this isn’t just luck. For example, if the brand drug’s average AUC is 100 units, the generic’s 90% confidence interval must stay between 80 and 125. If the interval dips to 78-92, it fails - even if the average is 85. That’s because real-world variability matters. The system is designed to catch even small, consistent differences.
Why 80-125%? Not 80-120%
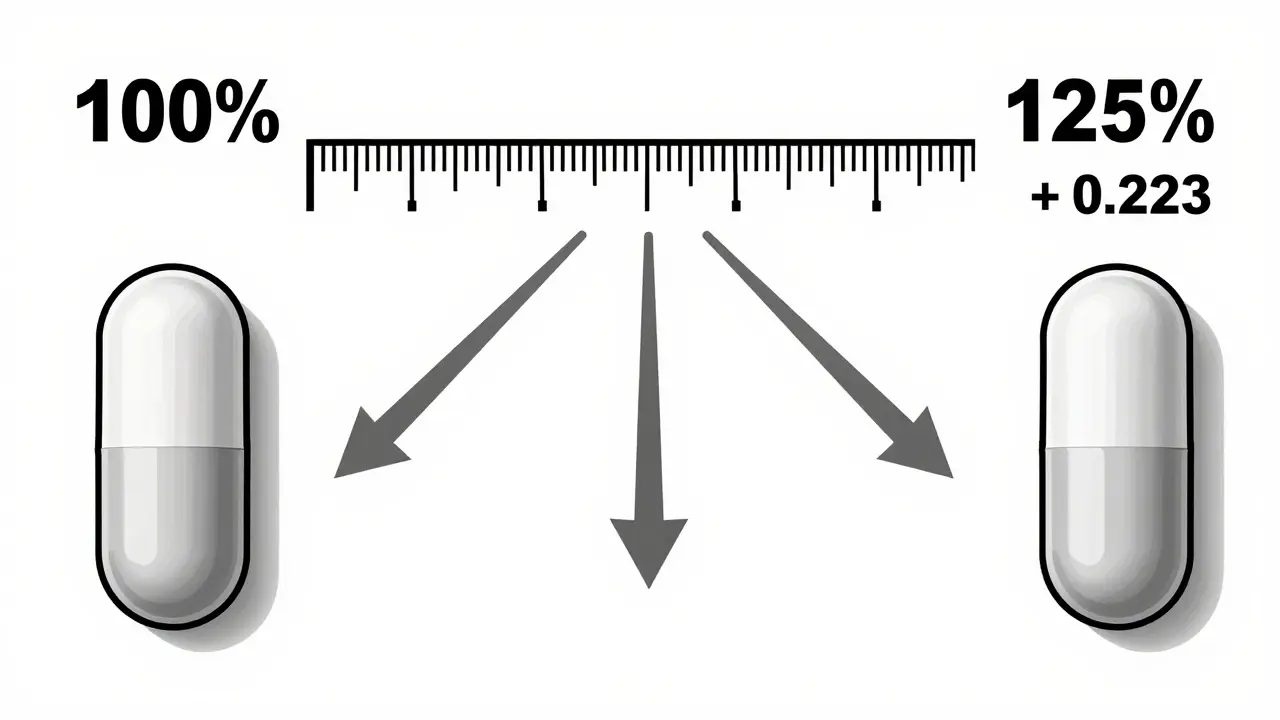
You might think the range should be symmetric - like 80-120%. But it’s not. That’s because drug absorption data doesn’t follow a normal bell curve. It follows a log-normal distribution. That means the math works better on a logarithmic scale.
Here’s the simple version: the natural log of 0.80 is -0.223, and the natural log of 1.25 is +0.223. So 80-125% is actually a ±22.3% spread on a log scale, centered at 100%. That’s why it looks uneven - it’s mathematically balanced for how drugs behave in the body. The FDA picked this range in 1992 after reviewing decades of data and expert input. It’s not arbitrary. It’s based on what’s clinically safe.
Real-World Performance: Are Generics Really That Close?
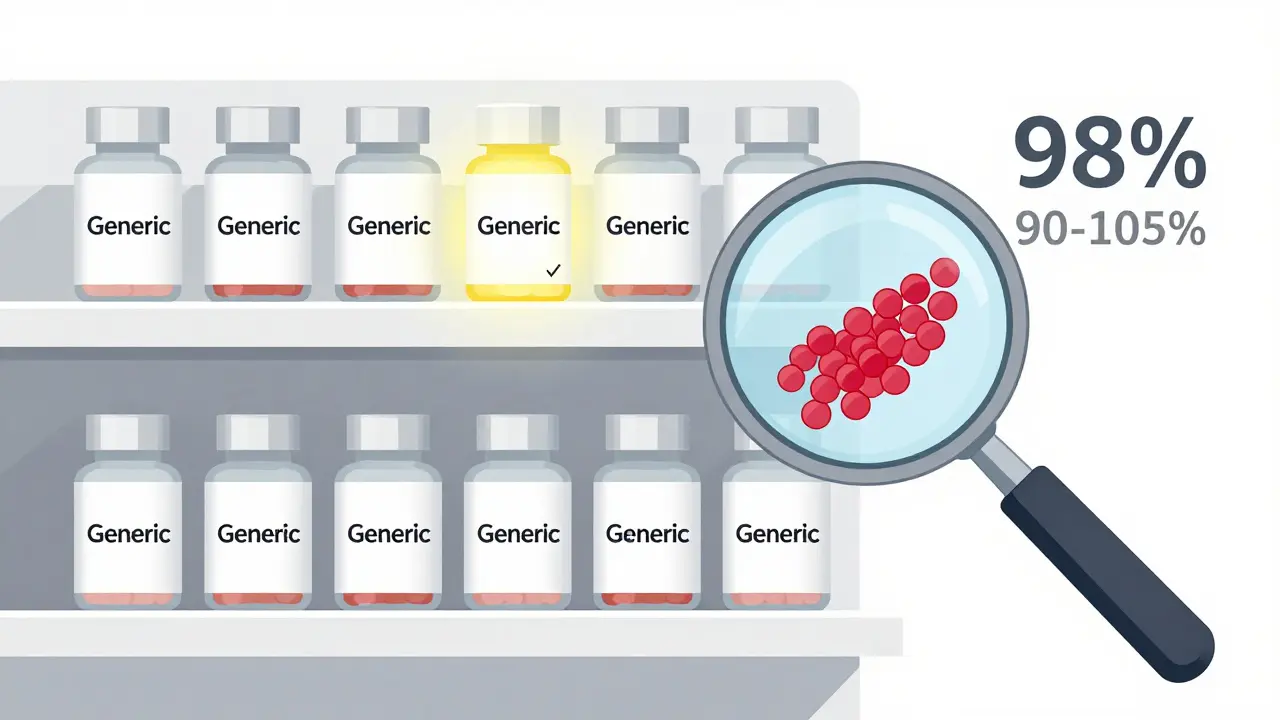
Here’s something surprising: most approved generics don’t even come close to the edges of that 80-125% range. A review of over 2,000 bioequivalence studies by the FDA between 2008 and 2012 found that 98% of generics had absorption rates between 90% and 105% of the brand drug. The average difference? Just 3.5%.
That’s not a fluke. A 2016 study in JAMA Internal Medicine looked at over 2 million patients taking generic versions of heart medications like statins and blood pressure drugs. The results? No difference in heart attacks, strokes, or hospitalizations compared to brand-name users. The same pattern holds for antidepressants, seizure meds, and thyroid drugs.
Even in high-stakes cases, like warfarin (a blood thinner), the FDA tightened the range to 90-111% - not because the original rule failed, but because even tiny differences matter more for drugs with a narrow therapeutic index. That’s the system working as designed: one size doesn’t fit all.
Why Do People Still Doubt Generics?
Despite the data, confusion runs deep. On pharmacy student forums and Reddit threads, you’ll still see comments like: “Generics can be 25% weaker.” That’s wrong - and dangerous. It’s a myth that sticks because the numbers look misleading. 80-125% sounds like a huge gap. But think of it like a speed limit: 80-125% isn’t saying your car can go 45 mph slower or 25 mph faster. It’s saying the engine’s output stays within a range that won’t make you crash or stall.
Pharmacists report that 78% of patients ask about this at least once a week. But when they explain it clearly - using real data, not jargon - 63% say patient concerns disappear. The FDA’s #GenericsWork campaign, which reached over a million people, focused on this exact misunderstanding. And it worked.
What About Complex Drugs?
Not all drugs are created equal. Tablets you swallow are straightforward. But inhalers, topical creams, or injectables? Their absorption depends on how they’re delivered - not just how much drug is in them. For those, the 80-125% rule isn’t enough.
That’s why the FDA now has special guidelines for complex generics. For example, a generic asthma inhaler must not only match the active ingredient but also replicate the particle size, spray pattern, and lung deposition. These aren’t tested with blood samples - they’re tested with machines that simulate breathing. The same goes for transdermal patches and nasal sprays.
The FDA’s 2023 draft guidance covers over 1,600 specific products with tailored rules. And by 2025, they plan to use computer modeling to predict how a drug will behave in the body - reducing the need for human trials in some cases. But for the vast majority of pills you take? The 80-125% rule still holds.
How This Impacts You
Here’s the bottom line: if your doctor prescribes a generic, you’re getting a drug that works the same way. The FDA approves over 800 generics every year. Each one had to pass this same rigorous test. And because of it, generics now make up 90% of all prescriptions in the U.S. - while costing only 23% of what brand drugs do.
That’s not just savings. That’s access. Millions of people rely on generics to afford their medications. If you’re worried about effectiveness, ask your pharmacist for the FDA’s bioequivalence summary. Or check the FDA’s Orange Book - it lists every approved generic and its reference brand. You’ll see the same active ingredient, the same dosage, and the same approval path.
There’s no evidence that generics fail more often. The FDA’s Sentinel Initiative tracked 200 million patient records from 2015 to 2020. For 94% of drugs, adverse event rates were identical between brand and generic versions. The few exceptions? Often linked to formulation changes, not the 80-125% rule.
What to Do If You’re Still Unsure
Still hesitant? Try this: if your generic works, keep taking it. If you notice a change - like new side effects or reduced effectiveness - talk to your doctor. But don’t assume it’s because it’s generic. More often, changes come from stress, diet, other meds, or even a different manufacturer’s filler ingredient. Those are rare, but they’re worth checking.
Pharmacists are trained to explain this. Ask them. Most have taken FDA-approved training courses. They’ve seen the data. They know the science. And they’re not selling you a cheaper version - they’re helping you get the same medicine at a fair price.
The 80-125% rule isn’t a loophole. It’s a guarantee. A scientifically proven, internationally accepted standard that keeps you safe, healthy, and able to afford your treatment. You don’t need to choose between cost and care. With generics, you get both.
Does the 80-125% rule mean generic drugs can have 25% less active ingredient?
No. The 80-125% rule does not refer to the amount of active ingredient in the tablet. It refers to how much of that ingredient your body absorbs into your bloodstream. Generic drugs must contain the same amount of active ingredient as the brand-name version. The rule ensures that the rate and extent of absorption are so similar that the drugs work the same way in your body.
Why is the range 80-125% and not 80-120%?
The range is based on logarithmic scaling, not a linear one. Drug absorption data follows a log-normal distribution, so the FDA uses natural logarithms to analyze it. The values 0.80 and 1.25 are symmetric on the log scale - ln(0.80) = -0.223 and ln(1.25) = +0.223. This creates a balanced, statistically valid range that reflects real biological variability. A linear 80-120% range would not accurately capture how drugs behave in the body.
Are generics just as safe as brand-name drugs?
Yes. The FDA requires generics to meet the same quality, strength, purity, and stability standards as brand-name drugs. Studies tracking over 200 million patient records found no significant difference in adverse events between generics and brand-name drugs for 94% of medications. The only exceptions involve rare formulation changes, not the bioequivalence standard itself.
Do generics work the same for all types of drugs?
For most oral tablets and capsules, yes. But for complex products like inhalers, topical creams, or injectables, absorption depends on how the drug is delivered - not just how much is in it. For these, the FDA uses special testing methods, sometimes including physical and performance tests instead of blood samples. The 80-125% rule still applies, but it’s adapted to the drug’s unique delivery system.
Why do some people say generics don’t work as well?
Misunderstanding the 80-125% rule is the biggest reason. Many think it means the drug can be 25% weaker or stronger - which is false. Other reasons include placebo effects, changes in inactive ingredients (like fillers), or unrelated health changes. When pharmacists explain the science clearly, patient concerns drop by over 60%. Real-world data shows no difference in outcomes between brand and generic drugs for most conditions.
Can I switch between different generic brands?
Yes. Every generic approved by the FDA must meet the same bioequivalence standards, regardless of manufacturer. Switching between different generic brands is safe for most people. For drugs with a narrow therapeutic index (like warfarin or levothyroxine), your doctor may recommend sticking to one brand - but that’s because small differences matter more, not because generics are unreliable.
How often does the FDA reject a generic drug for not meeting bioequivalence?
About 32% of generic applications are initially incomplete or fail bioequivalence testing. That’s because the standards are strict. Companies must prove their product matches the brand within the 90% confidence interval for both AUC and Cmax. Many fail on first try due to poor formulation or manufacturing inconsistencies. The fact that so many are rejected - and then resubmitted successfully - shows the system works to protect patients.
Do other countries use the same 80-125% rule?
Yes. The European Medicines Agency (EMA), Health Canada, and over 50 other countries use the exact same 80-125% bioequivalence standard with a 90% confidence interval. This global alignment ensures that generics approved anywhere meet the same safety and effectiveness benchmarks. It’s not just an American rule - it’s the international gold standard.

Joanna Domżalska
January 26, 2026 AT 10:04So what you're saying is we're just trusting math that was made by people who probably never had to take this stuff themselves? I mean, if my body absorbs it differently, who cares what the FDA says? I feel different on generics. That's real data.
Faisal Mohamed
January 27, 2026 AT 14:51Log-normal distribution ftw 📈🤯 The 80-125% range isn't arbitrary-it's the *geometric mean* of pharmacokinetic variability! AUC and Cmax are log-transformed because drug absorption is multiplicative, not additive. Most folks don't get that the confidence interval is on the *log scale*, not linear. This is why the FDA doesn't just wing it. 🧪🧪
Josh josh
January 28, 2026 AT 09:54bro the whole 80-125 thing is just a trick to make generics look good but honestly ive switched between brands and sometimes the generic makes me feel like a zombie and the brand makes me feel like im 20 again
bella nash
January 29, 2026 AT 14:33It is imperative to underscore that the bioequivalence threshold is grounded in statistically rigorous methodology, predicated upon a 90% confidence interval, and validated across multiple clinical cohorts. The regulatory framework is not capricious; it is, in fact, exceptionally conservative.
SWAPNIL SIDAM
January 31, 2026 AT 06:17My uncle in India takes generic blood pressure pills for 10 years. No hospital. No problem. The science is real. People just scared of cheap things. In our village, we say: if it works, it’s not fake.
TONY ADAMS
February 1, 2026 AT 09:30you think the FDA cares about you? they just want big pharma to stop charging $1000 for a pill that costs 2 cents to make. the 80-125 rule is a loophole so they can say 'we checked' while letting companies cut corners on fillers. i got seizures on a generic. lucky i caught it before i died.
Napoleon Huere
February 2, 2026 AT 15:59Think about it: the body doesn't care if a pill is branded or generic. It only cares about the molecule. If the molecule is identical and the absorption profile is statistically equivalent, then the experience should be too. The fear isn't scientific-it's psychological. We fear what we don't understand, and we distrust what we can't see.
Robin Van Emous
February 4, 2026 AT 08:49I get why people are nervous. I used to be too. But after working in a pharmacy for 12 years, I’ve seen it over and over: same drug, same effect. The only time someone had an issue? They switched from one generic to another generic, and the filler changed. Not the medicine. The chalk. That’s it. The system works. Just talk to your pharmacist. They’re the real heroes here.
Angie Thompson
February 5, 2026 AT 20:40OMG I just learned this and my mind is BLOWN 🤯 Like, I thought generics were just knockoffs but nooo-this is like two identical engines with different paint jobs and one has a slightly different air filter… but still runs the same! And the FDA is like a super strict mechanic checking every bolt. I’m switching back to generics now. 💪❤️
shivam utkresth
February 7, 2026 AT 19:37In India, we call this 'jugaad'-making smart workarounds with limited resources. The 80-125% rule? That’s jugaad with science. It’s not cutting corners, it’s cutting costs without cutting corners. Millions of us depend on this. Don’t let fear stop you from living. The science? Solid. The savings? Life-changing.