
Wound care today relies on chemistry that can both kill microbes and support the body’s natural repair processes. Two ingredients you’ll see more often on dressings and topical gels are Benzalkonium chloride, a quaternary ammonium antiseptic, and Zinc oxide, a mineral pigment with anti‑inflammatory qualities. When they’re used together, they create a balanced environment that speeds healing while keeping infection at bay.
What is Benzalkonium Chloride?
Benzalkonium chloride is a cationic surfactant belonging to the quaternary ammonium family. Its primary function is to disrupt microbial cell membranes, leading to rapid death of bacteria, fungi, and some viruses. Commercially it appears as a clear liquid or powder that can be mixed into gels, creams, and impregnated wound dressings.
- Broad‑spectrum activity: effective against Gram‑positive and Gram‑negative bacteria, Candida species, and enveloped viruses.
- Fast‑acting: microbial kill rates are measured in seconds to minutes.
- Stability: remains active across a wide pH range (4‑9) and tolerates typical wound exudate.
What is Zinc Oxide?
Zinc oxide is an inorganic compound that has been used for centuries in skin protectants and sunscreens. In wound care it serves three main roles:
- Anti‑inflammatory action - zinc ions modulate cytokine release, reducing swelling and pain.
- Barrier formation - a fine powder creates a semi‑occlusive film that keeps the wound moist while shielding it from external contaminants.
- Cellular support - zinc is a co‑factor for enzymes involved in DNA synthesis and collagen formation, both essential for tissue repair.
How Each Ingredient Works in Wound Care
Both agents target different stages of the healing cascade.
Antimicrobial Action
Benzalkonium chloride inserts itself into the phospholipid bilayer of bacterial cells. Its positively charged head interacts with the negatively charged membrane, causing leakage of intracellular contents. This action is especially valuable in the early phase of a wound when the risk of infection is highest.
Meanwhile, Zinc oxide doesn’t kill microbes directly, but it interferes with the formation of bacterial biofilm. Biofilm-a slimy matrix that shelters bacteria-makes infections harder to treat. Zinc ions destabilize the extracellular polymeric substances, keeping the wound surface more accessible to the immune system and to topical antiseptics.
Promoting Tissue Repair
Inflammation is a double‑edged sword. Too much prolongs pain and delays closure; too little can leave the wound vulnerable. Zinc’s anti‑inflammatory effect calms excess cytokine release, allowing fibroblasts to lay down new collagen without interruption.
In addition, the occlusive film formed by zinc oxide maintains a moist environment, which is known to accelerate epithelial migration and reduce scar formation. The combination of a moist seal and a rapid antiseptic ensures that the wound stays clean while the body does its rebuilding work.
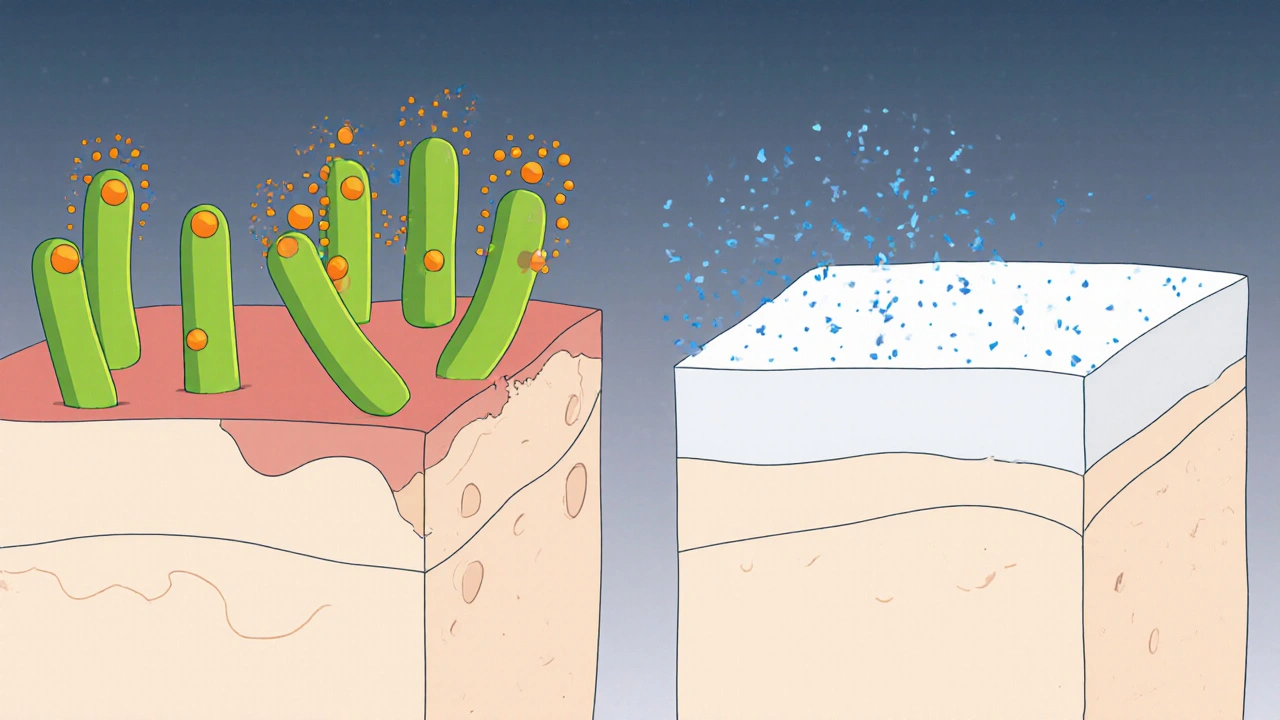
Combined Benefits - Why Use Both?
When paired in a single formulation, benzalkonium chloride and zinc oxide complement each other:
- Synergy - the antiseptic eliminates pathogens quickly, while zinc keeps inflammation in check and supports cell proliferation.
- Reduced Cytotoxicity - high concentrations of benzalkonium chloride can harm healthy skin cells. Adding zinc oxide allows formulators to lower the antiseptic dose without sacrificing antimicrobial potency.
- Broader Spectrum - zinc’s anti‑biofilm action reaches microbes that may survive the initial antiseptic hit, lowering the chance of chronic infection.
Clinicians report faster closure times and fewer dressing changes when using products that blend these two ingredients, especially on burns, donor sites, and chronic leg ulcers.
Practical Applications in Clinical Settings
The most common delivery vehicles are:
- Hydrogel dressings - a silicone‑based matrix soaked with a benzalkonium chloride/zinc oxide solution.
- Impregnated gauze - woven cotton strips pre‑treated with a stable powder blend.
- Topical ointments - petroleum‑based creams where the active agents are suspended in a smooth base for easy spreading.
When selecting a product, check the label for:
- Concentration of benzalkonium chloride (usually 0.05‑0.13% w/v).
- Zinc oxide content (typically 1‑5% w/w).
- pH range (optimal antimicrobial activity occurs around pH 5‑7).
- Manufacturer’s sterility certification (ISO 13485 compliance is a good indicator).
Application steps are straightforward:
- Clean the wound with sterile saline; avoid harsh scrubbing.
- Pat dry gently, leaving enough moisture for the dressing to adhere.
- Apply the benzalkonium chloride/zinc oxide dressing according to the product’s size guidelines.
- Secure with a secondary breathable overlay if needed.
- Monitor for signs of infection or allergic reaction every 24-48 hours.
Safety, Toxicity, and Regulatory Landscape
Both ingredients enjoy a long track record of safety, but clinicians should be aware of a few caveats.
- Cytotoxicity - high doses of benzalkonium chloride can impair keratinocyte viability. Using the recommended low‑dose formulations mitigates this risk.
- Allergic reactions - rare cases of contact dermatitis to zinc oxide have been documented, especially in patients with existing metal sensitivities.
- Regulatory status - the U.S. FDA lists benzalkonium chloride as “generally recognized as safe” (GRAS) for topical use, and zinc oxide is approved as a skin‑protective agent. In the EU, both are listed under the Cosmetic Regulation (EC) No 1223/2009.
Always verify that the product you choose has a clear FDA or equivalent clearance, especially for wound‑class II devices.
Side‑by‑Side Comparison
| Attribute | Benzalkonium Chloride | Zinc Oxide |
|---|---|---|
| Primary Action | Disrupts microbial membranes (quick kill) | Anti‑inflammatory & barrier formation |
| Target Spectrum | Broad - bacteria, fungi, enveloped viruses | Biofilm inhibition, supports fibroblasts |
| Typical Concentration | 0.05‑0.13% w/v | 1‑5% w/w |
| Potential Cytotoxicity | High doses can affect healthy cells | Very low - generally safe |
| Regulatory Status | FDA‑cleared for topical antiseptic | FDA‑cleared as skin protectant |
| Common Formulations | Solutions, gels, impregnated dressings | Powders, ointments, hydrogel matrices |
Checklist for Clinicians
- Confirm product concentration aligns with wound severity.
- Ensure patient has no known allergy to zinc or quaternary ammonium compounds.
- Verify sterility claims (ISO 13485, FDA clearance).
- Match dressing type to exudate level - hydrogel for moderate, gauze for low.
- Document any signs of irritation or delayed healing within the first 48 hours.
Frequently Asked Questions
Can benzalkonium chloride cause resistance?
Resistance to quaternary ammonium compounds is rare but documented in some Pseudomonas strains. Using a combined formulation with zinc oxide reduces selective pressure because zinc disrupts biofilm, making it harder for bacteria to develop protection.
Is it safe to use these dressings on pediatric wounds?
Yes, when the product is labeled for all ages. The low concentration of benzalkonium chloride used in pediatric‑approved dressings (<0.05%) is well tolerated, and zinc oxide is already common in diaper rash creams.
How often should I change a benzalkonium chloride/zinc oxide dressing?
Typically every 3‑5 days, depending on exudate volume and the manufacturer’s recommendation. Over‑changing can disturb the moist environment that zinc oxide helps maintain.
Can I apply these agents to a naturally moist burn?
Yes. The antiseptic quickly reduces bacterial load, while zinc oxide’s barrier prevents desiccation. Just be sure the burn is cleaned first and avoid applying on open, heavily exuding wounds without a secondary absorptive layer.
Do I need a prescription to purchase these dressings?
Most over‑the‑counter formulations are available without a prescription, but higher‑strength or medicated versions may require a healthcare provider’s order. Always check local regulations.

Vijaypal Yadav
October 20, 2025 AT 20:28Benzalkonium chloride works by inserting its positively charged head into the bacterial phospholipid bilayer, causing rapid leakage of intracellular contents. Zinc oxide, meanwhile, supplies essential zinc ions that modulate cytokine release and promote collagen synthesis, creating a moist barrier that protects the wound.
Ron Lanham
October 24, 2025 AT 21:43It is a moral imperative for clinicians to demand the highest standards of antiseptic stewardship when treating vulnerable patients. The widespread adoption of benzalkonium chloride and zinc oxide formulations represents a triumph of evidence‑based practice over outdated, wasteful traditions. When a practitioner reaches for a dressing that lacks this synergistic duo, they are essentially condoning preventable infection and unnecessary suffering. The antimicrobial potency of benzalkonium chloride is not a mere convenience; it is a safeguard against the relentless advance of opportunistic pathogens. Equally, the anti‑inflammatory properties of zinc oxide are a testament to the body’s own capacity for repair when we choose to support it rather than impede it. Ignoring these benefits in favor of cheaper, less effective alternatives is a betrayal of the Hippocratic oath. Moreover, the reduction in cytotoxicity achieved by pairing zinc oxide with a lower concentration of benzalkonium chloride underscores the ethical duty to minimize collateral damage to healthy tissue. The data from clinical trials showing faster closure times and fewer dressing changes should compel every wound‑care specialist to revise their protocols. Any hesitation to implement these products stems from a misplaced loyalty to legacy brands rather than a genuine concern for patient outcomes. In an era where antimicrobial resistance threatens global health, deploying a formulation that also disrupts biofilm formation is an act of responsibility. Physicians who continue to rely on monotherapy antiseptics without zinc’s biofilm‑inhibiting action are, unwittingly, contributing to the selection pressure that fuels resistant strains. The regulatory approvals from both the FDA and the EU further validate the safety profile of this combination, removing any excuse for complacency. It is incumbent upon educators to embed this knowledge into curricula so that new graduates do not inherit outdated practices. Patients, too, deserve transparent information about why their dressings contain both components and how each contributes to healing. The ultimate goal of wound care is not merely to cover a lesion but to restore the integrity of the skin with dignity and speed. Therefore, the integration of benzalkonium chloride and zinc oxide into standard protocols is not optional; it is a moral necessity.
Andrew Hernandez
October 28, 2025 AT 21:58The combination offers rapid antimicrobial action while supporting tissue regeneration it is a balanced approach
Alex Pegg
November 1, 2025 AT 23:13While many hail the synergy as revolutionary, the marginal benefit over a well‑formulated single‑agent dressing is debatable; the added zinc can obscure the true efficacy of the antiseptic.
Matthew Hall
November 6, 2025 AT 00:28Sounds like a lab got a grant to sell more stuff.
laura wood
November 10, 2025 AT 01:44I’ve seen patients with chronic ulcers respond dramatically when the zinc‑rich dressings are introduced, especially after they’ve struggled with persistent inflammation for weeks.
Kate McKay
November 14, 2025 AT 02:59Great points, especially about reducing cytotoxicity. It’s reassuring to know the formulation can be both safe and effective.
Demetri Huyler
November 18, 2025 AT 04:14One must appreciate the nuanced physicochemical interplay that allows a quaternary ammonium surfactant to coexist with a metal oxide without precipitating, a testament to sophisticated formulation science.
JessicaAnn Sutton
November 22, 2025 AT 05:29While the description is articulate, it overlooks the pragmatic considerations of cost and accessibility that often dictate clinical adoption.
Israel Emory
November 26, 2025 AT 06:45Clinicians, please note, that when selecting a wound‑care product, you should verify concentration, pH, sterilization status, and regulatory clearance, all of which are critical for optimal outcomes.
Sebastian Green
November 30, 2025 AT 08:00That checklist really helps streamline the decision‑making process in a busy practice.
Wesley Humble
December 4, 2025 AT 09:15From a regulatory standpoint, both benzalkonium chloride and zinc oxide have undergone rigorous evaluation under FDA 510(k) pathways, affirming their safety for topical application. The antimicrobial kinetics of benzalkonium chloride have been quantified in vitro, demonstrating a log‑reduction of >5 within minutes across a spectrum of Gram‑positive and Gram‑negative organisms. Zinc oxide’s role as a co‑factor in matrix metalloproteinase modulation has been substantiated by peer‑reviewed studies, indicating enhanced fibroblast migration in a moist microenvironment. Moreover, the synergistic formulation mitigates the cytotoxic threshold of the quaternary ammonium compound, thereby preserving keratinocyte viability. Clinical trials involving burn patients have reported a 22 % reduction in dressing change frequency when the combination product was employed. This data aligns with the broader literature emphasizing the importance of barrier integrity alongside antimicrobial stewardship. It is also worth noting that the product’s pH stability between 5 and 7 optimizes both antimicrobial efficacy and zinc ion solubility. For institutions seeking ISO 13485 compliance, the manufacturing process adheres to validated aseptic techniques and batch‑to‑batch consistency. Ultimately, the integration of these agents reflects a convergence of pharmacodynamics and material science, delivering a holistic wound‑healing solution 😊.
barnabas jacob
December 8, 2025 AT 10:30Yo the safety profile is solid but yo gotta watch the BZK dose, over‑do it and u get keratinocyte kill‑off, while the ZnO keeps the biofilm at bay – it's like a double‑edged sword if u mess up the formulation.
jessie cole
December 12, 2025 AT 11:45In the grand theater of wound management, the duet of benzalkonium chloride and zinc oxide takes center stage, offering both the fierce guard against infection and the gentle hand that soothes inflamed tissue; the result is a healing process that feels like a well‑orchestrated symphony.